Chemical Properties of Candle Wax
Candle curing is the process of continuous hardening of wax to disperse fragrance oils evenly throughout the blend. Upon applying extremely high pressure several hundred atmospheres to.

Candle Flame Color Zones Chemistry Candles Candle Flames
C Both processes A and B are chemical changes.

. The mass of the substance is altered during a chemical change. Excess oil tends to pool in the candle if it contains poorly mixed fragrance or theres simply too much in the wax. A burning candle is the best example of physical and chemical change.
The blubber oil of the whale is about 66 wax. Science - 5th. Baking soda and vinegar.
Heat some sugar crystals in a test tube over a flame. Call Our Instant Help Desk on 234 814 010 7220 and Get Your Complete Project Material Instantly. The heat from the flaming wick vaporizes the wax a hydrocarbon which in turn reacts with oxygen in the air to release carbon dioxide and water.
The changes in chemical change are irreversible and permanent. End up with candle wax and ashes in the ear. In the demonstration burning of the candle is a chemical change while conversion of the candle to wax is a physical change.
The first industrially practical polyethylene synthesis diazomethane is a notoriously unstable substance that is generally avoided in industrial application was again accidentally discovered in 1933 by Eric Fawcett and Reginald Gibson at the Imperial Chemical Industries ICI works in Northwich England. For example when we light a candle it will burn until the wax burns out. When a candle burns both physical and chemical changes take place.
There is a tool used for the removal of earwax known as Ototek Loop Ear Wax Removal Tool. It is composed mostly of wax esters chiefly cetyl palmitate and a smaller proportion of triglycerides. HOW TO GET YOUR COMPLETE CHEMICAL ENGINEERING PROJECT INSTANTLY.
When a candle burns both physical and chemical changes occur. Give another example of a familiar process in which both the chemical and physical changes take place. State the differences between chemical and physical changes.
The properties of these starch products are determined both by their botanical origin and further chemical andor physical modifications. Submit the 3 topics to your Supervisor for Approval. Over the process heat and light energy is given out.
Select 3 CHEMICAL ENGINEERING Project Topics of your choice from the list above. It is a chemical change. Survey concludes that the candling process involves a deposit of candle wax within the ear and it has to be removed by the doctor itself.
This change is not reversible. Choose the one that is not a sign of a chemical reaction occurring. How to pour NatureWax candles.
Raw spermaceti is liquid within the head of the sperm whale and is said to have a smell similar to raw milk. D None of these processes is a chemical change. Properties of Matter Vocabulary.
Hence the burning of a candle is a chemical change. It is primarily concerned with the exploitation of chemical reactions on a commercial scale. The wick of the candle gets changed to a black mass.
This branch is a very important activity in chemical engineering. It is not possible to i recover the burnt wax again ii recover the thread again. Which word does not belong.
It reveals that chemical change cannot be reversed by changing or altering the experimental changes. This means that this type of reaction does not need any external. This type of combustion occurs spontaneously.
Unlike other toothed whales most of the carbon chains in the wax esters are relatively long C 10 C 22. Anaerobic bacteria digest animal waste and produce biogas ChangeA. The combustion occurs as long as the fuel is available.
A ProcessA is a chemical change. As time passes we can observe that the candle changes to wax. Also called clean combustion complete combustion is the oxidation of a hydrocarbon that produces only carbon dioxide and waterAn example of clean combustion would be burning a wax candle.
Different wax and fragrance oil combinations may require more or less time to achieve an optimal state. Either the mass is added or removed. B ProcessB is a chemical change.
This activity probably more than any other sets chemical engineering apart as a distinct. Its goal is the successful design and operation of chemical reactors. During a chemical change energy changes occur.
The following statements pertain to these changes. Granular native starch when heated in an aqueous environment gelatinizes to produce a viscous. We do not get wax back from carbon carbon dioxide and water by cooling them or any other method.
A large amount of heat and light energy is produced in this type of reaction. If you cover the candle with a jar it will extinguish. All project materials on this website are well researched.
Take a candle and light it. Chemical changes create a new product. Ii A chemical change cannot be easily reversed.
Prevent candle cracking and pull away. The burning of wax in a candle is a chemical change producing carbon carbon dioxide and water vapour. The biogas is then burnt as fuel ChangeB.
Melting of wax vapourisation of.
What Really Happens To The Wax As Your Candle Burns Quora

Melting Candle Wax To Explore States Of Matter States Of Matter Melting Candles Science Activities For Kids
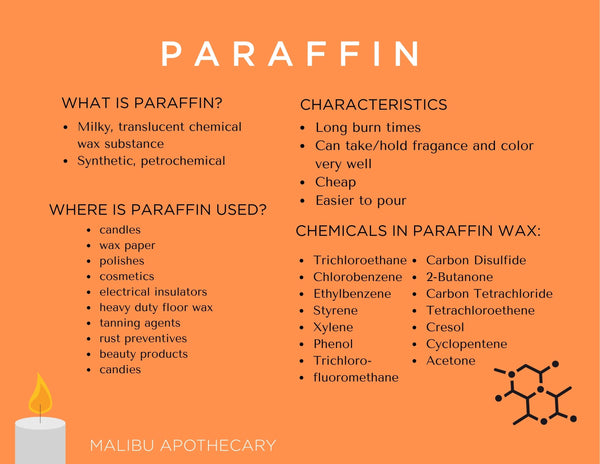

No comments for "Chemical Properties of Candle Wax"
Post a Comment